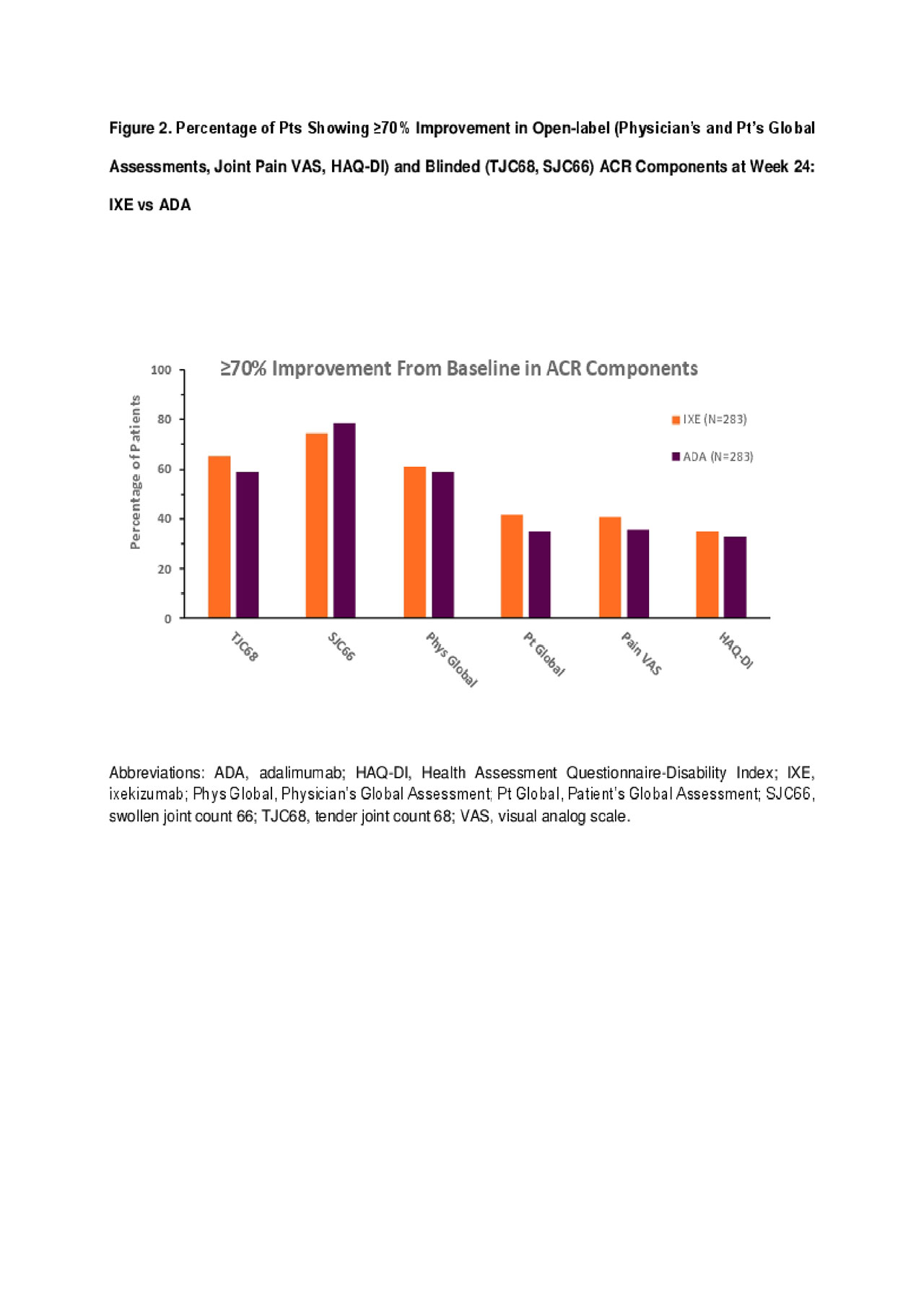
Ixekizumab Demonstrates Improvement Comparable to Adalimumab Across ACR Components in Biologic-Naïve Patients with Psoriatic Arthritis - ACR Meeting Abstracts

Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)

PDF) Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: Results from the randomised, controlled and open-label phases of UNCOVER-3

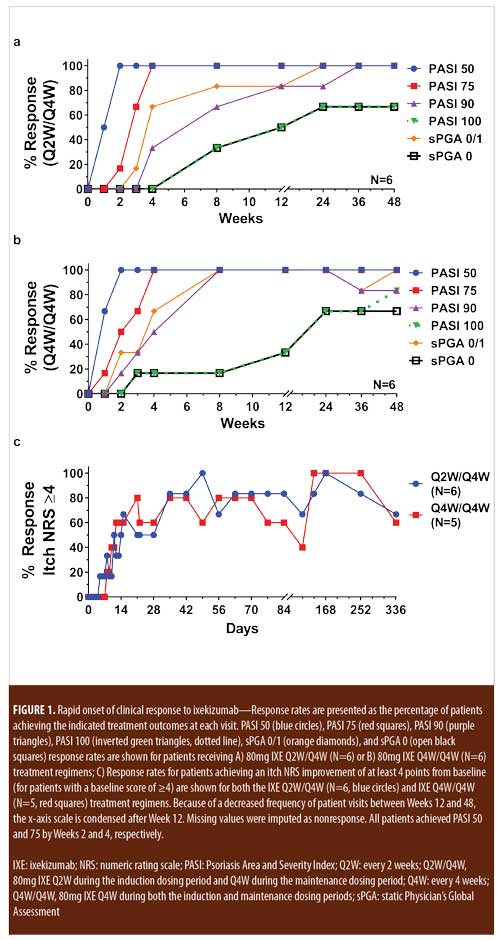
A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis - ScienceDirect

Lilly's Taltz® (ixekizumab) is the First IL-17A Antagonist to Receive U.S. FDA Approval for the Treatment of Non-Radiographic Axial Spondyloarthritis (nr-axSpA)
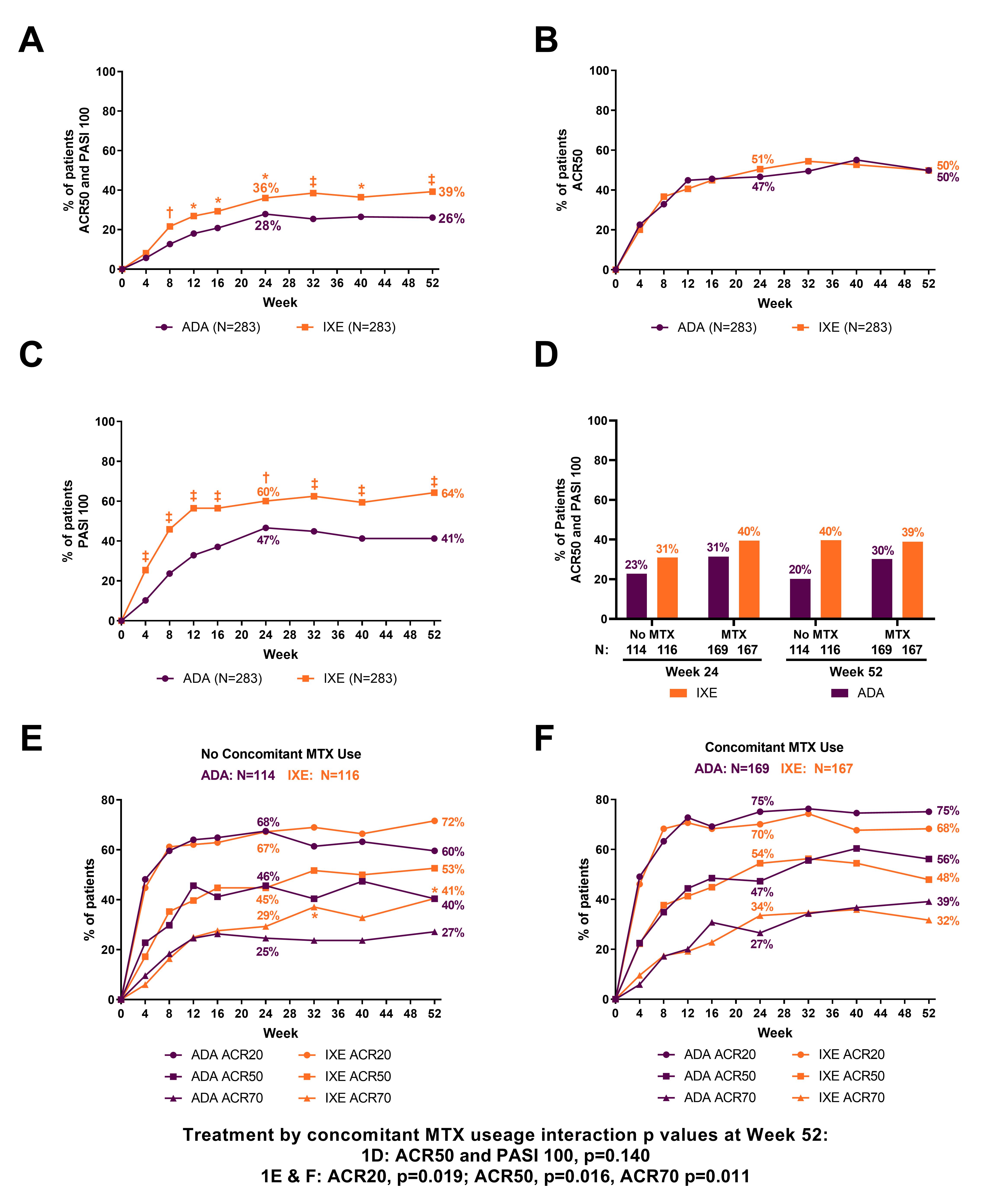
A Head-to-Head Comparison of Ixekizumab and Adalimumab in Biologic-Naïve Patients with Active Psoriatic Arthritis: Efficacy and Safety Outcomes from a Randomized, Open-Label, Blinded Assessor Study Through 52 Weeks - ACR Meeting Abstracts

Fillable Online accessdata fda LABEL. TALTZ (ixekizumab) injection, Eli Lilly and Company - accessdata fda Fax Email Print - pdfFiller

Figure 3. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology

Long‐term efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis: subgroup analyses of an open‐label, phase 3 study (UNCOVER‐J) - Okubo - 2019 - Journal of the

Study design for administration of ixekizumab via a prefilled syringe... | Download Scientific Diagram

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect
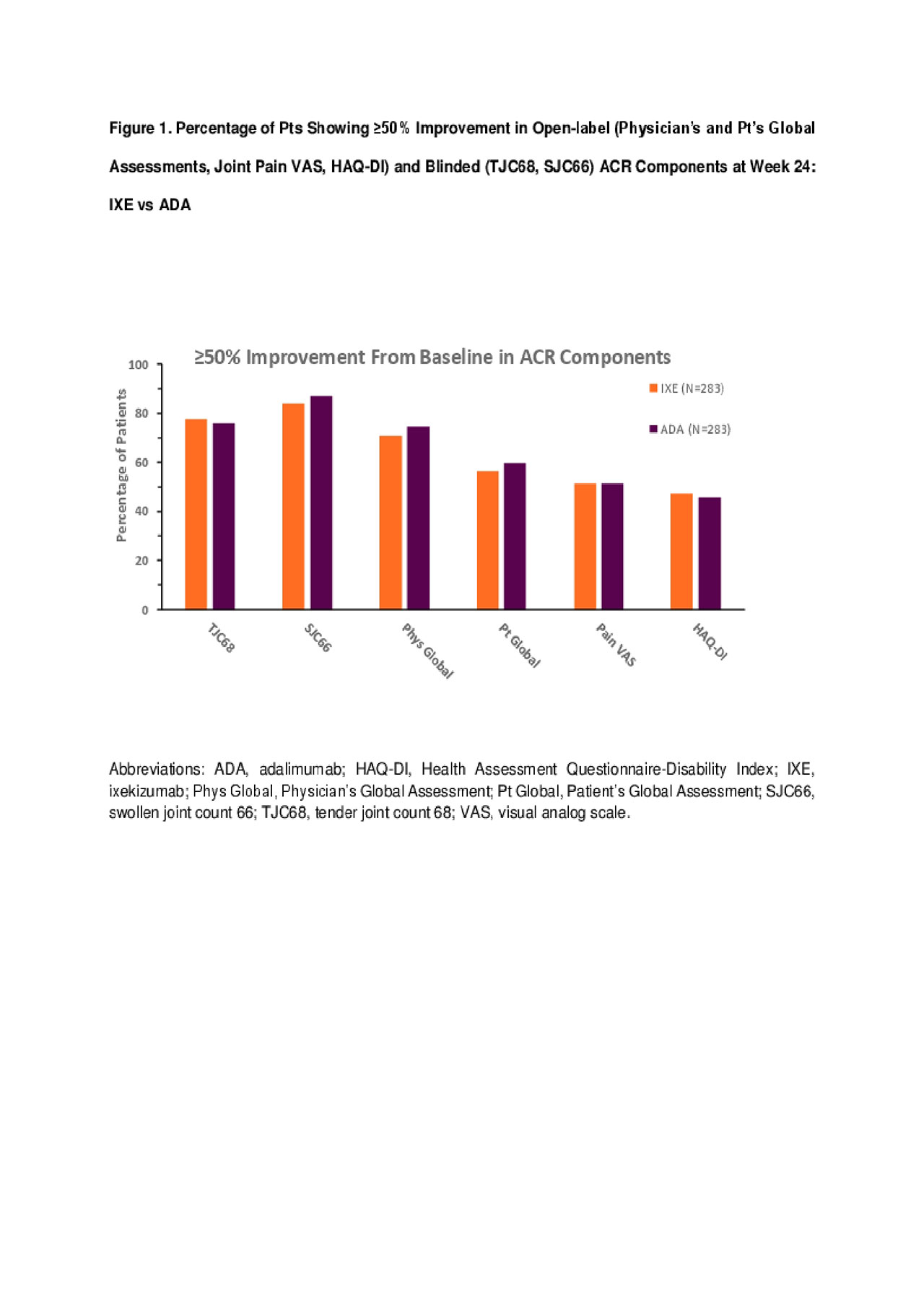
Ixekizumab Demonstrates Improvement Comparable to Adalimumab Across ACR Components in Biologic-Naïve Patients with Psoriatic Arthritis - ACR Meeting Abstracts

Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)

Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial - The Lancet

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - Journal of the American Academy of Dermatology