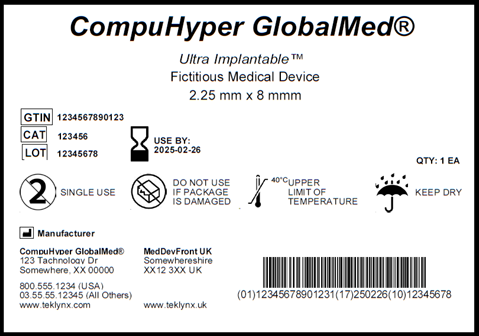
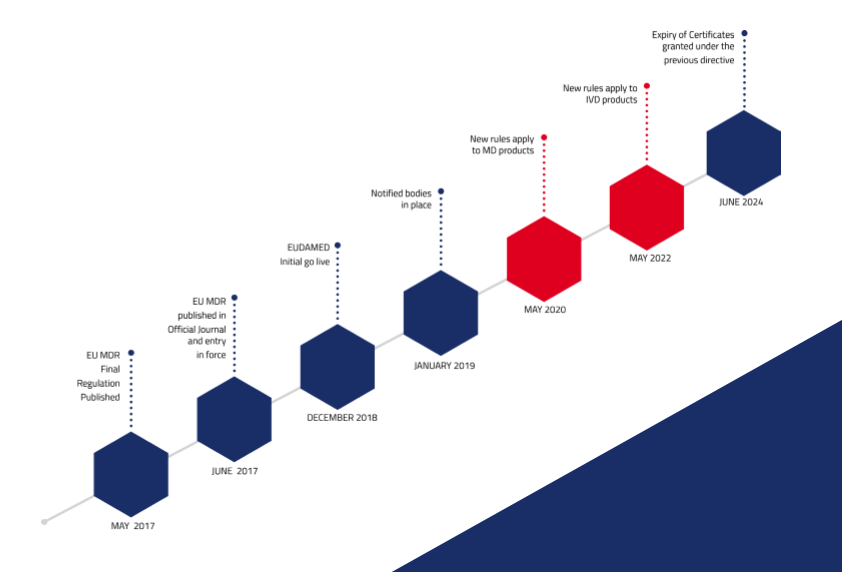
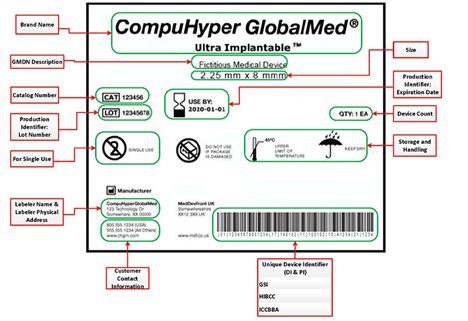
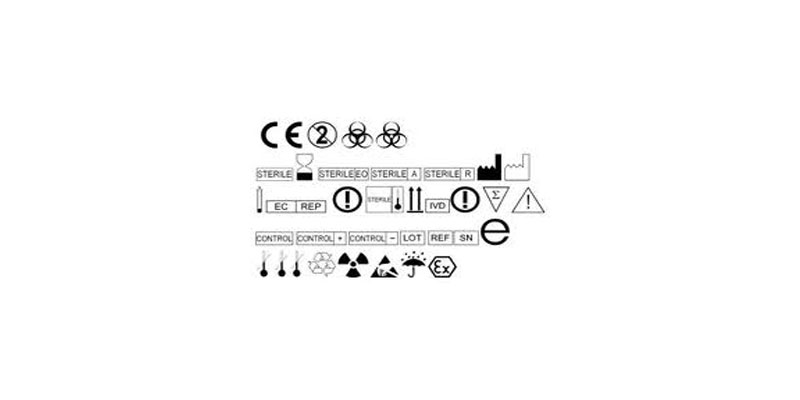
THE EU MDR LABELLING JOURNEY: BEST PRACTICES FOR NAVIGATING THE LATEST MEDICAL DEVICE LABELLING REQUIREMENTS - CSOFT Health Sciences

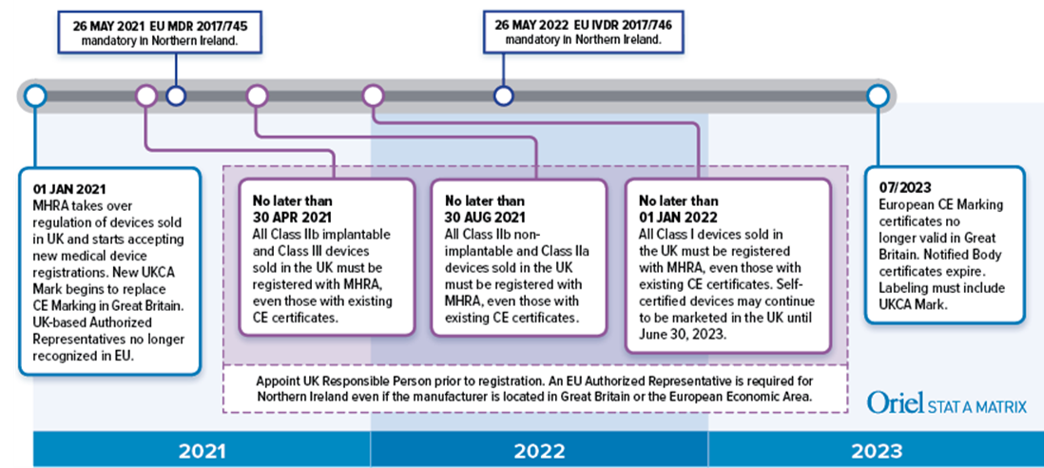
EU MDR - Medical Device Labeling Changes & Challenges - Regulatory, Clinical Consulting Services to Biopharma & Medical Device Companies