![QMS for Software as a Medical Device [SaMD] Lessons Learned from a Quality Perspective - PDF Free Download QMS for Software as a Medical Device [SaMD] Lessons Learned from a Quality Perspective - PDF Free Download](https://docplayer.net/docs-images/42/17134037/images/page_2.jpg)
QMS for Software as a Medical Device [SaMD] Lessons Learned from a Quality Perspective - PDF Free Download

No more paper medical instructions - dokspot: helping MedTech companies go paperless — OneMillionSparks

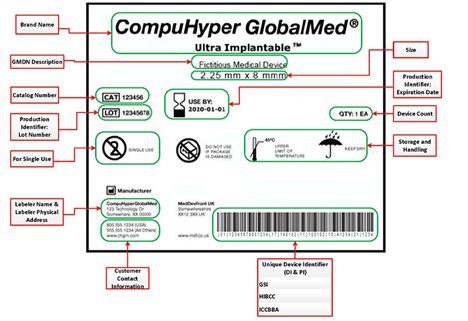
Unique device identification and traceability for medical software: A major challenge for manufacturers in an ever-evolving marketplace - ScienceDirect