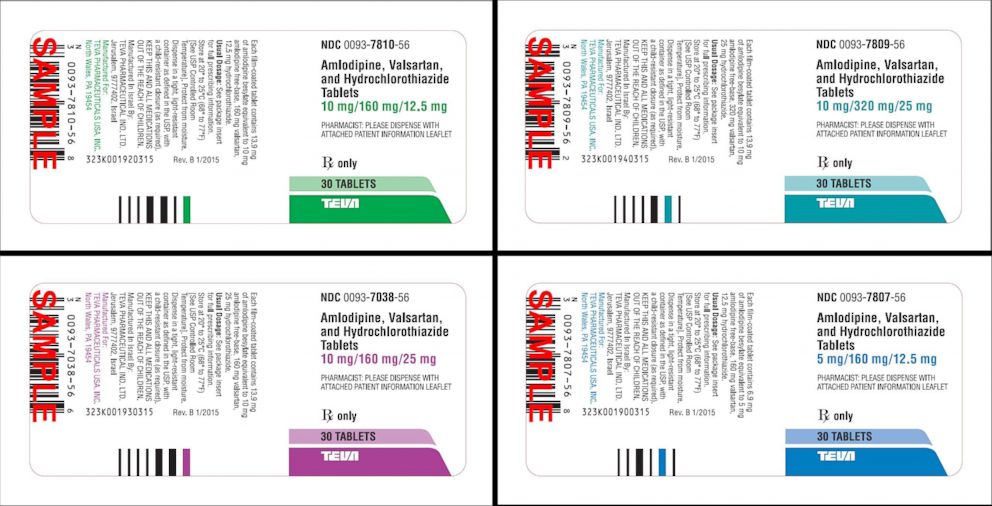
Teva Pharmaceuticals USA Issues Voluntary Nationwide Recall of all Amlodipine/Valsartan Combination Tablets and Amlodipine/Valsartan/Hydrochlorothiazide Combination Tablets that are Within Expiry | FDA

FDA joins EU in seeking recall of certain Chinese-made valsartan products over potential cancer risk | FiercePharma
Teva Issues Recall of Tainted Blood Pressure Medicine, the Latest Global Recall for Valsartan | BioSpace